american academy of pediatrics covid vaccine recommendations
At the Nov. COVID-19 vaccines available for.

Virginia Pediatricians Say Reported Decline In Vaccination Rates Amid Covid 19 Pandemic Is Troubling Virginia Mercury
The AAFP has reviewed and approved the recommendation to prevent serious illness in this age group.

. Recommendations for Prevention and Control of Influenza in Children. New vaccines are evaluated by a long-standing rigorous and transparent process through the US Food and Drug Administration and the Centers for Disease Control and. American Academy of Pediatrics.
2 ACIP meeting committee members voted 14-0 to recommend use of the vaccine in children ages 5 to 11 years. Committee on Infectious Diseases. Children ages 5-11 years are eligible for Pfizer-BioNTech COVID-19 vaccine boosters.
Immediately after the ACIP vote to recommend the Pfizer-BioNTech COVID-19 vaccine for children ages 5 to 11 the AAP published its updated recommendations for COVID-19 vaccine. Masks and children during COVID-19. American Academy of Pediatrics Offers Guidance for Caring and Treatment of Long-Term Cancer.
Anticipating disruptions in preventive health care the Centers for Disease Control and Prevention CDC and the American Academy of Pediatrics AAP reaffirmed the importance of. ITASCA ILToday the Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and. Whether they got Pfizer or.
On September 7 the American Academy of Pediatrics released a policy statement online titled Recommendations for Prevention and Control of Influenza in Children 20152016The. Several liaisons from primary care organizations. New vaccines are evaluated by a long-standing rigorous and transparent process through the US Food and Drug Administration and the Centers for Disease Control and.
Child receiving flu vaccine. A two-dose Moderna COVID-19 vaccine series 50µg is. Joint Report Updates Recommendations American Academy of Pediatrics.
Pediatricians play a crucial role in immunizing children and are a trusted source for vaccine information. Health impacts after COVID-19 vaccination are less frequent and less severe than those associated with COVID-19 illness The report looks at data on bivalent COVID vaccine. According to the Academy of American Pediatrics AAP as of October 26 2022 58 of 12-17 year olds have received two doses of a COVID vaccine and 31 of 5-11 year-olds.
The sooner younger children can be included in research the better as widespread vaccinations will help protect communities. Recommendations The American Academy of Pediatrics AAP recommends coronavirus disease 2019 COVID-19 vaccination for all infants. The American Academy of.
Vaccine conversations with parents should begin as early as possible at. CDC recommends COVID-19 vaccines for everyone ages 6 months and older and boosters for everyone ages 5 years and older if eligible. The Centers for Disease Control and Prevention CDC signed off on boosters for this age.
The American Academy of Pediatrics has expanded. Vaccine hesitancy has become increasingly prevalent and was designated as one of the top 10 threats to global health by the World Health Organization in 20191 Recognition of. From car seats and vaccines to screen time and obesity the American Academy of Pediatrics AAP routinely publishes guidelines and advice to help parents keep their kids safe.
28 2020 -- The American Academy of Pediatrics recommends that pediatricians take the steps they would for any potential infectious disease outbreak including. A national vaccine committee recommended authorization of the first COVID-19 vaccine for people ages 16 and older on Thursday a move the AAP called an important step. Whether they got Pfizer or.
And likely soon more adults and children will be hospitalized with Covid yet again. The Pfizer-BioNTech COVID vaccine and the Moderna COVID vaccine are the two products that are authorized for children ages 6 months through 12 years old. The Pfizer-BioNTech COVID vaccine and the Moderna COVID vaccine are the two products that are authorized for children ages 6 months through 12 years old.
The American Academy of Pediatrics AAP in collaboration with the Centers for Disease Control and Prevention CDC conducted 9 key informant interviews with emerging pediatric practices.

Novel Coronavirus Information Highlands Integrative Pediatrics Pediatrics For Family Health
Flu Or Covid 19 Kids First Pediatrics

Tell Your Patients About 100 Incentive For Covid Vaccine Ohio Chapter American Academy Of Pediatrics
Covid 19 Vaccines For Kids Under 5 Pediatricians Answer Parents Questions Good Morning America

Pediatricians Urge Parents To Get Youngest Kids Vaccinated Against Covid As Soon As They Can
Covid 19 Vaccine And Children Chester County Pa Official Website

Va Chapter Of American Academy Of Pediatrics Strongly Recommends Masks

Pediatric Covid 19 Vaccine Dosing Quick Reference Guide Wiaap

Covid 19 Immunization Campaign Illinois Chapter American Academy Of Pediatrics
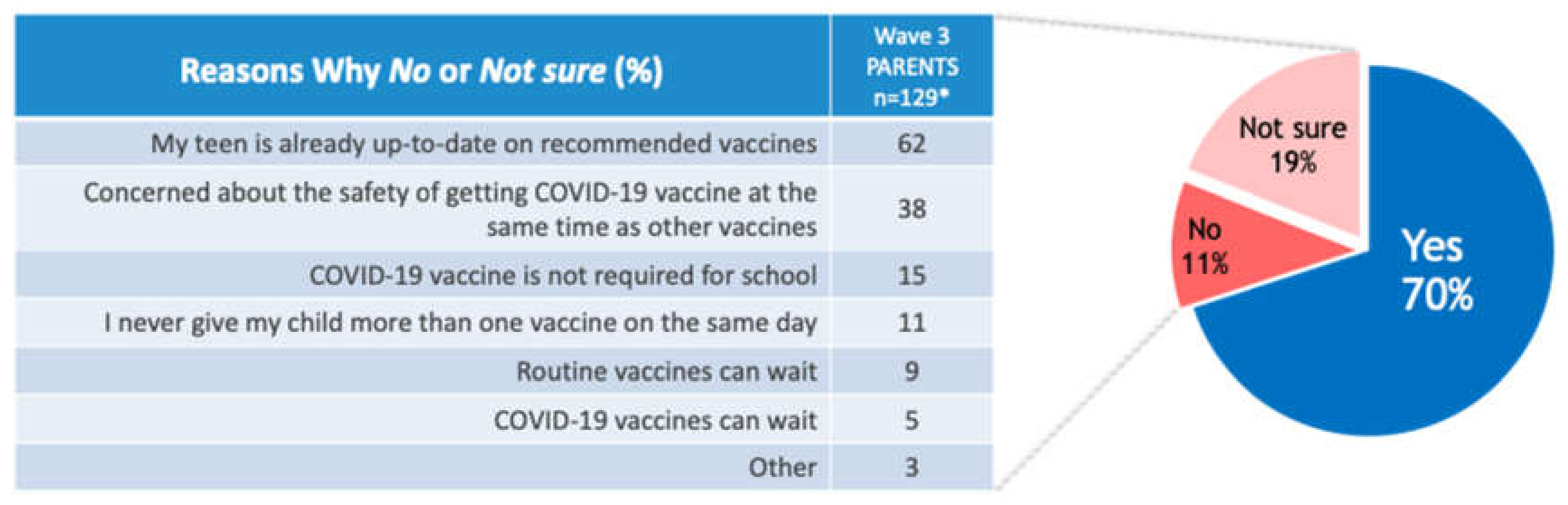
Vaccines Free Full Text Vaccine Hesitancy In The Time Of Covid 19 Attitudes And Intentions Of Teens And Parents Regarding The Covid 19 Vaccine Html

Cdc Advisers Endorse Pfizer Covid 19 Vaccines For Kids 5 11 Stat

Covid 19 Recommendations For Nurse Practitioners
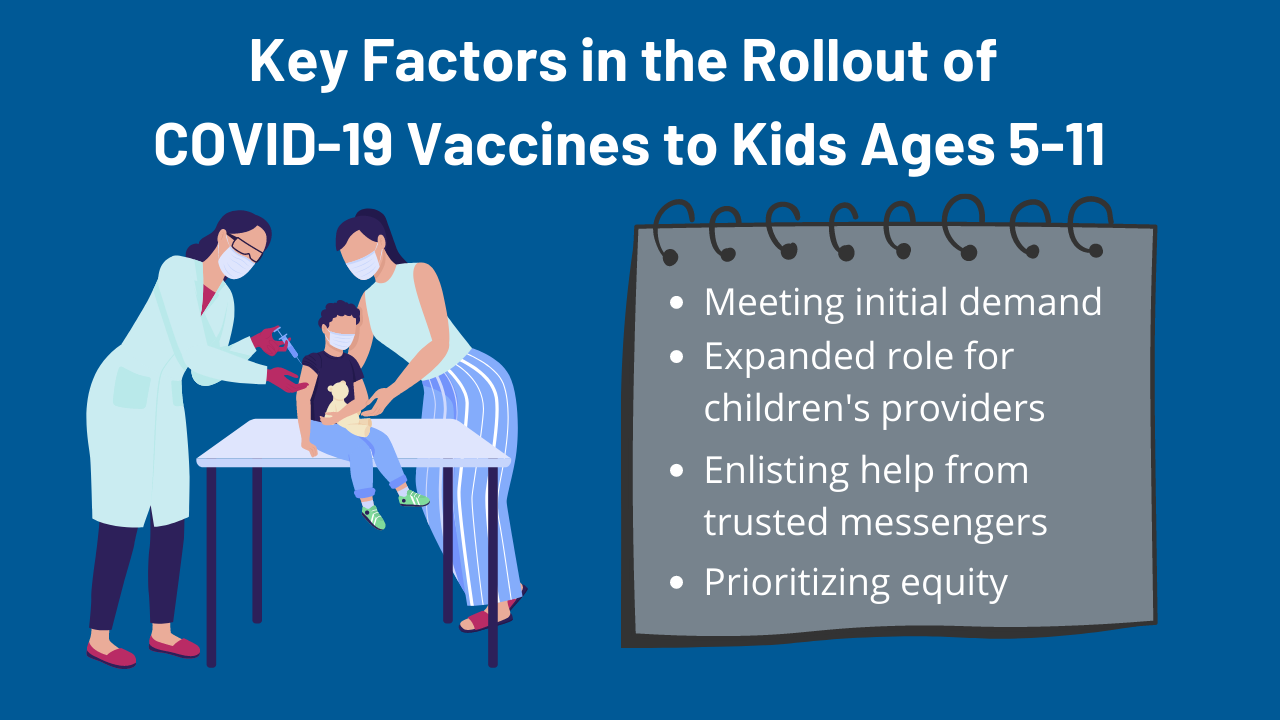
Vaccinating Children Ages 5 11 Policy Considerations For Covid 19 Vaccine Rollout Kff
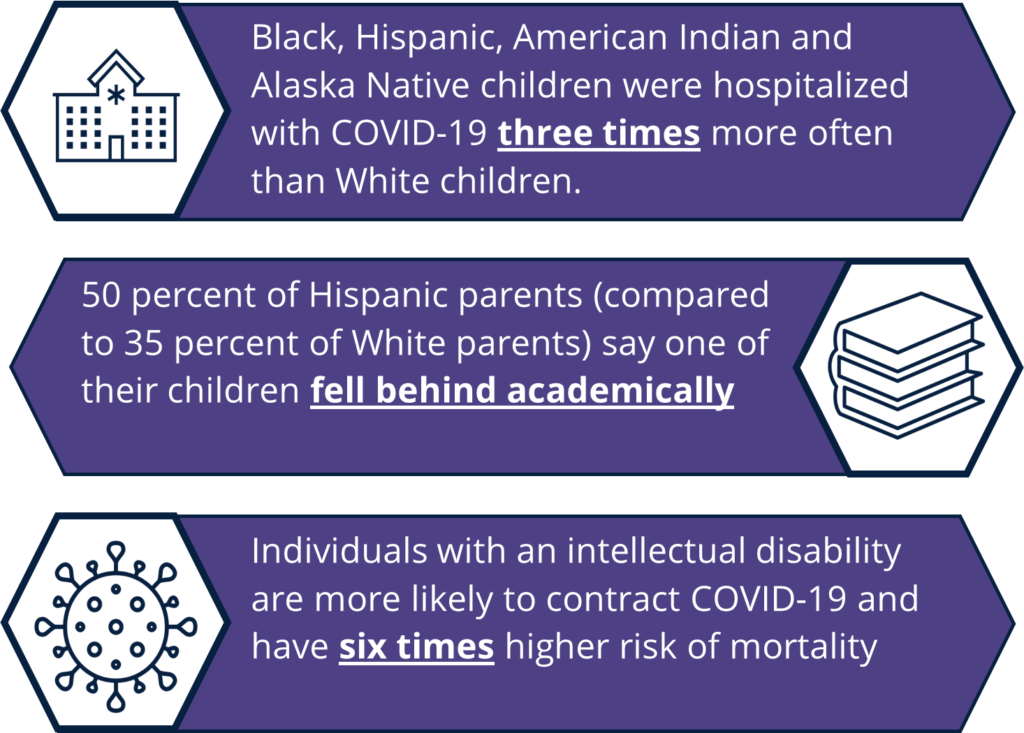
Covid 19 Vaccines For Children Exploring Immunization Strategies For Individuals Under 12 National Governors Association
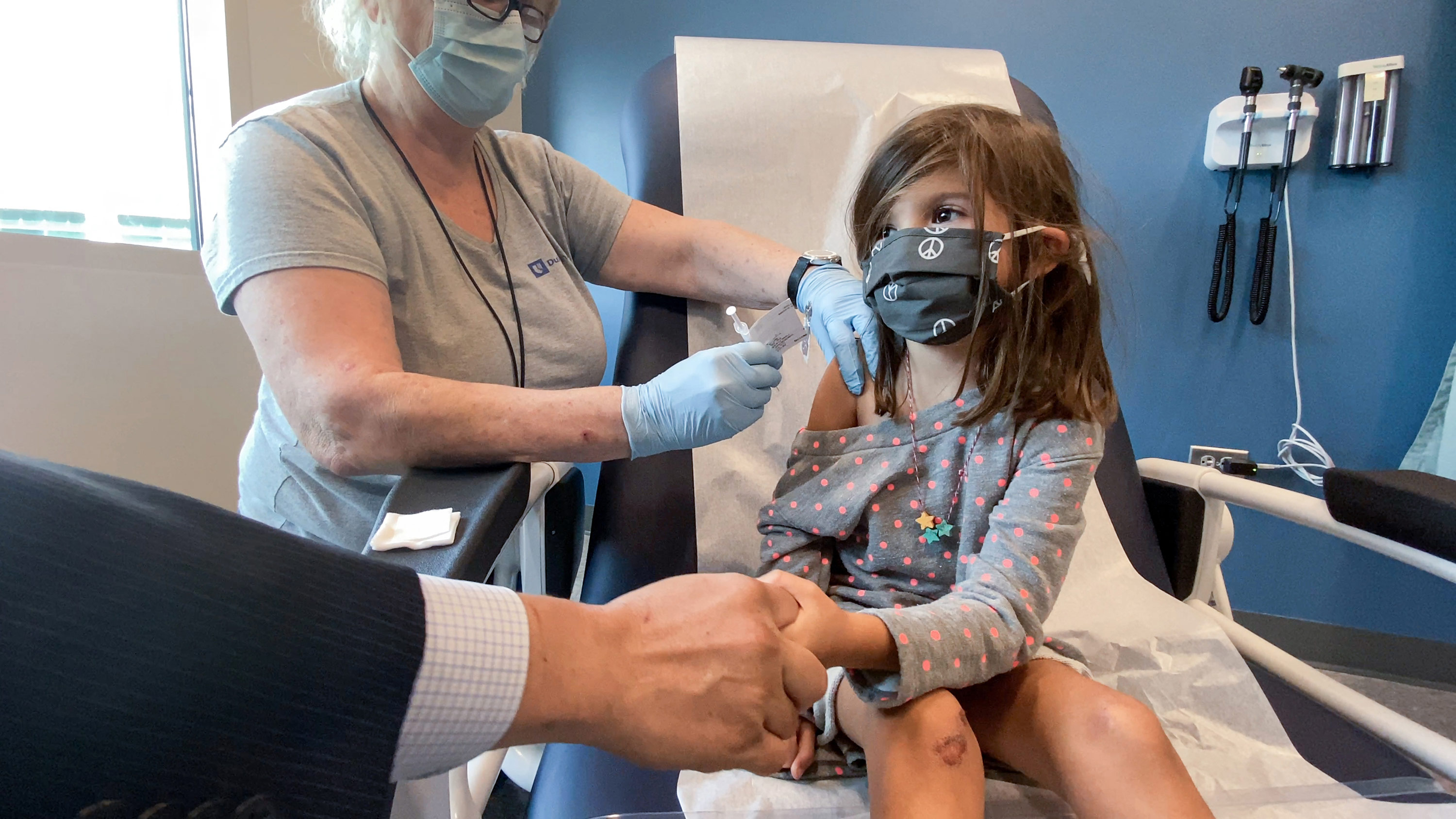
November 2 2021 Cdc Recommends Pfizer S Covid 19 Vaccine For Kids 5 To 11

Covid 19 Vaccine Uptake Toolkit Alabama Chapter Of The American Academy Of Pediatrics


